
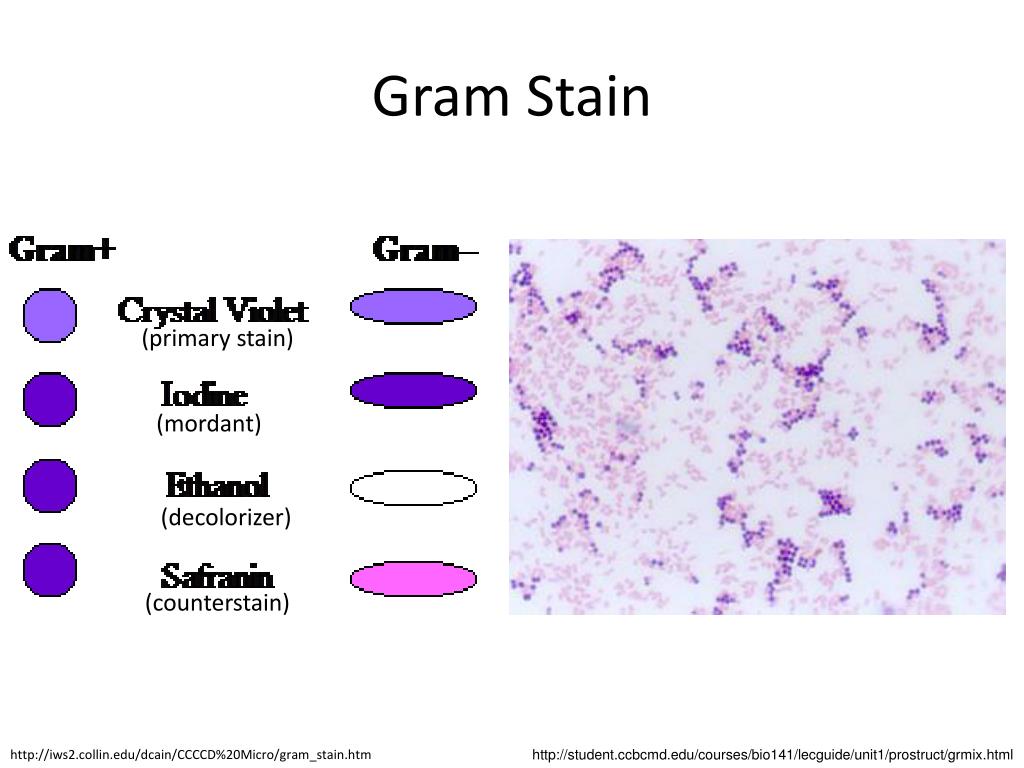
Metallic iron deposits, or inert iron oxide seen in tissues because of industrial exposure, are not positive when treated with acid ferrocyanide solutions. This precipitate will not occur when the slides are stained at room temperature. However, the use of heat will sometimes cause a fine blue precipitate to form on both the tissue section and slide. A similar result is obtained if the acid ferrocyanide solution is heated to 60☌ in a water bath, oven, or microwave oven. Both hemoglobin and myoglobin are examples of such protein complexes and, if treated with hydrogen peroxide (100 vol), the iron is released and can then be demonstrated using Perls' Prussian blue reaction ( Fig. This is because the iron is tightly bound within a protein complex. Certain types of iron found in tissues are not demonstrable using traditional techniques. Fixatives that contain acids but no formalin can remove hemosiderin or alter it in such a way that reactions for iron are negative. In unfixed tissue, hemosiderin is insoluble in alkalis but freely soluble in strong acid solutions after fixation in formalin, it is slowly soluble in dilute acids, especially oxalic acid. Churukian, in Theory and Practice of Histological Techniques (Sixth Edition), 2008 Demonstration of hemosiderin and iron Each method gives its own instructions for preparing the solution, but many of them can substitute for each other.Charles J. In any case, there should not be a strong ammonia smell in the finished solution, just a faint hint of it.
STAIN PRECIPITATE FREE
This ensures there is no residual free ammonium hydroxide in the solution. Some even stipulate that a small amount of aqueous silver nitrate should be added to the final solution after all silver oxide has redissolved, then the solution filtered to remove any silver oxide particles which the addition produces. This care is necessary because any excess of ammonia makes the solution more alkaline and less effective. Most are clear that only the minimum amount of ammonium hydroxide should be added to redissolve the silver oxide, stipulating that it should be added drop by drop from a Pasteur pipette and that the final stage should be done slowly, and may involve merely waving the pipette over the surface of the solution to allow ammonia fumes to dissolve in it. Some variations even wash the silver oxide with distilled water before adding ammonium hydroxide to redissolve it, claiming that this gives a cleaner background. There are many variations for producing these solutions, differing by the volume and concentration of silver nitrate used and the chemical used to produce the initial precipitate of silver oxide, whether this be ammonium hydroxide, sodium hydroxide or sodium carbonate. Do not return it to the stock ammonium hydroxide bottle. Discard any ammonium hydroxide that is unused. The solution in the beaker may then be used to make the ammoniacal silver solution. It is advisable to open a stock bottle of ammonium hydroxide only long enough to pour some into a beaker, then to recap it securely. The resulting ammoniacal silver solutions may not work properly and the impregnations may fail. Such depleted solutions should not be used to make ammoniacal silver solutions. Ammonium hydroxide solutions give off ammonia when exposed to air and old solutions may have lost a distinct amount of their ammonium hydroxide content as a consequence. The ammonium hydroxide must be saturated and should have a specific gravity of 0.88. with a doubled "m", but they all refer to the same thing. The "diamin-" in the names may be spelled as "diammin-", i.e. It is variously known as silver diaminohydroxide, silver diamine (with or without the word " hydroxide" added), diamine silver, Tollen's reagent or, most commonly in histology, ammoniacal silver solution, and contains compounds based on the following structure - OH.
On the addition of more ammonium hydroxide, the precipitate redissolves by forming a complex with the ammonia. Their preparation is based on the fact that ammonium hydroxide, when added to a simple aqueous silver solution, causes a precipitate of silver oxide to form.

These are made by adding strong ammonium hydroxide (concentrated ammonia, specific gravity 0.88) to an aqueous silver nitrate solution. Many silver impregnations employ ammoniacal silver solutions, sometimes referred to as silver diaminohydroxide solutions.


 0 kommentar(er)
0 kommentar(er)
